Through Appili’s unique approach to drug development, we have created a pipeline containing a deliberate mix of market and close-to-market opportunities that have the potential to provide near-term value with cutting-edge programs that we believe have the potential to transform how we treat many infectious diseases, addressing significant unmet medical needs in patient care.
This approach allows Appili to seek productive collaborations with industry partners and federal government agencies whose objectives align with ours, while pursuing non-dilutive funding sources specifically earmarked for infectious diseases development programs.
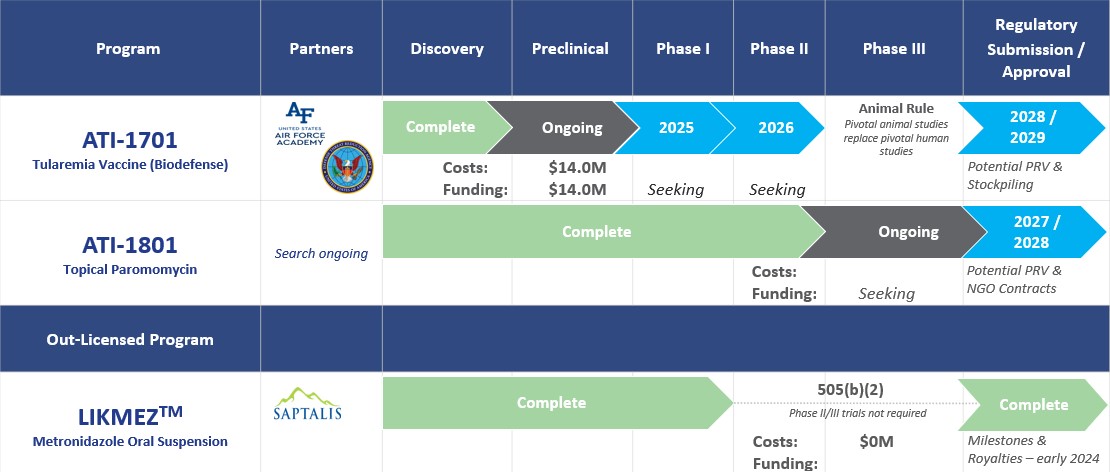

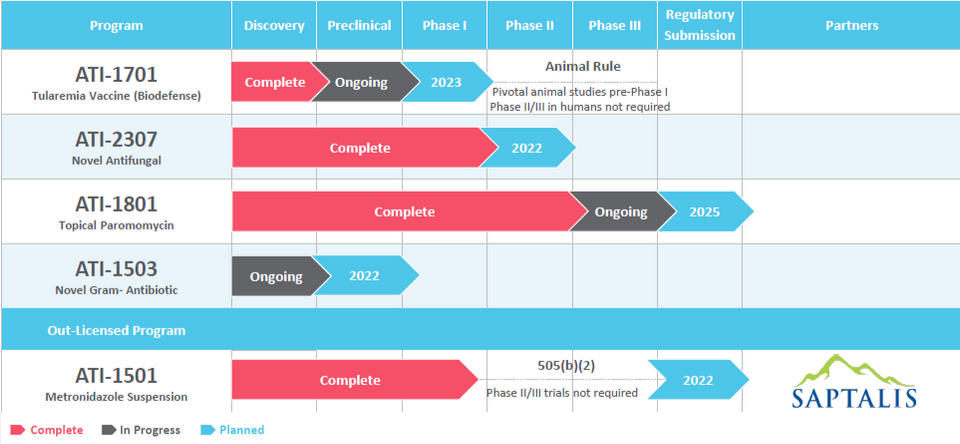
Once issued, a PRV can be used by its holder to accelerate the review of a subsequent drug submission. PRVs are transferrable and the secondary market for PRVs is well established with over 30 transactions reported publicly with recent transactions often exceeding US$100 million.